Details of the Drug
General Information of Drug (ID: DMJHLSD)
| Drug Name |
Famciclovir
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
FCV; Famciclovirum; Famvir; Oravir; BRL 42810; IN1338; Anti-Farnesyl Rabbit pAb; BRL-42810; Famciclovirum [INN-Latin]; Famvir (TN); Famciclovir [USAN:BAN:INN]; Famciclovir (JAN/USAN/INN); [2-(acetyloxymethyl)-4-(2-aminopurin-9-yl)butyl] acetate; Diacetyl 6-deoxy-9-(4-hydroxy-3-hydroxymethyl-but-1-yl)guanine; 1,3-Propanediol, 2-(2-(2-amino-9H-purin-9-yl)ethyl)-, diacetate (ester); 2-(2-(2-Amino-9H-purin-9-yl)ethyl)-1,3-propanediol diacetate (ester); 2-(2-(2-amino-9H-purin-9-yl)ethyl)-1,3-propanediol diacetate; 2-(acetoxymethyl)-4-(2-amino-4,5-dihydro-9H-purin-9-yl)butyl acetate; 2-[(acetyloxy)methyl]-4-(2-amino-9H-purin-9-yl)butyl acetate; 2-[2-(2-amino-9H-purin-9-yl)ethyl]-1,3-propanediol diacetate; 9-(4-acetoxy-3-(acetoxymethyl)but-1-yl)-2-aminopurine; 9-[4-acetoxy-3-(acetoxymethyl)but-1-yl]-2-aminopurine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Human Herpes Virus
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
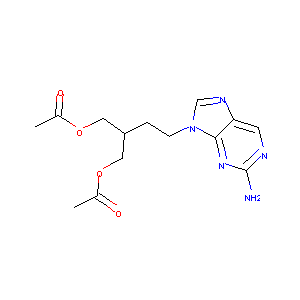 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 321.33 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Famciclovir (Comorbidity)
|
|||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Famciclovir FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Penciclovir cream--improved topical treatment for herpes simplex infections. Skin Pharmacol Physiol. 2004 Sep-Oct;17(5):214-8. | ||||
| 8 | Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002 Dec;303(3):1029-37. | ||||
| 9 | Evidence that famciclovir (BRL 42810) and its associated metabolites do not inhibit the 6 beta-hydroxylation of testosterone in human liver microsomes. Drug Metab Dispos. 1993 Jan-Feb;21(1):18-23. | ||||
| 10 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
